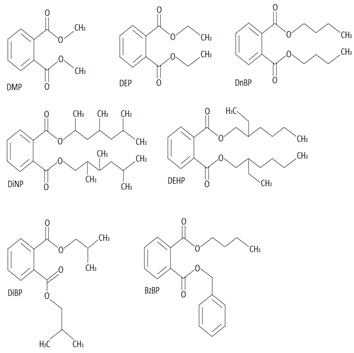
| Common phthalates and their uses in industry [7] | ||||||||||||||||||||||||||||
|


| Common phthalates and their uses in industry [7] | ||||||||||||||||||||||||||||
|
 While electronics companies have begun scaling back the use of phthalates under public pressure and increasing regulation, production changes are only part of the solution. The pathways that electronics take when they have reached the end of their life-cycle can have as much effect as the materials used in their construction. Even as companies reduce the levels of toxic materials in their products, large amounts of e-waste are improperly recycled or simply disposed of in poorer and less regulated parts of the world [24].
While electronics companies have begun scaling back the use of phthalates under public pressure and increasing regulation, production changes are only part of the solution. The pathways that electronics take when they have reached the end of their life-cycle can have as much effect as the materials used in their construction. Even as companies reduce the levels of toxic materials in their products, large amounts of e-waste are improperly recycled or simply disposed of in poorer and less regulated parts of the world [24].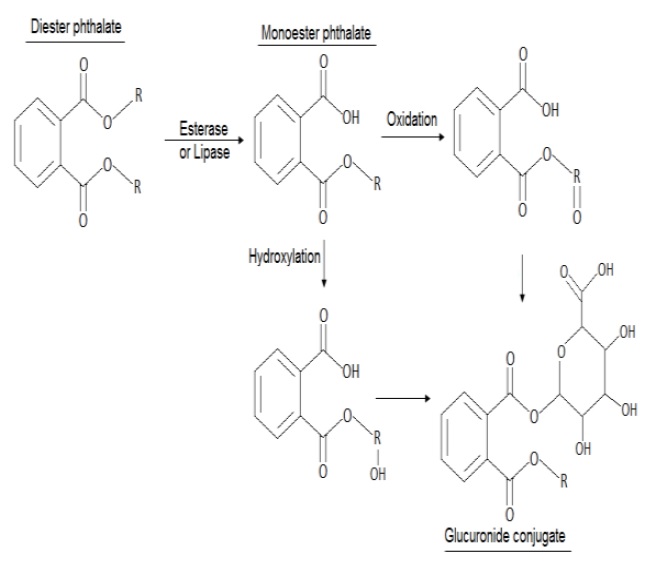
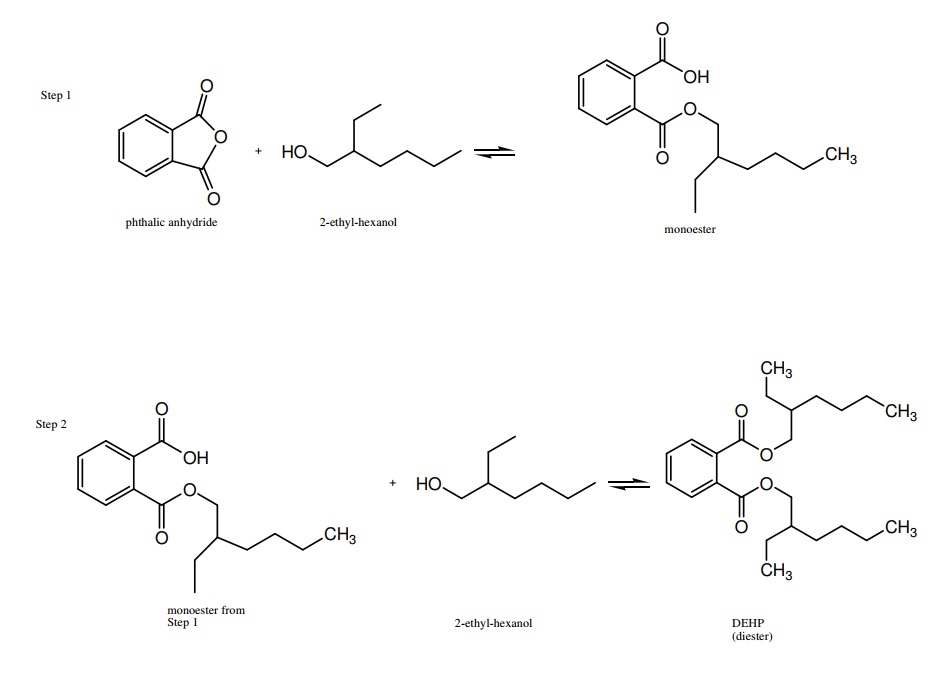
 Dewalque et al. [16] evaluated phthalate concentrations of five different phthalates in Belgium—DEP, DnBP, DiBP, BBzP, and DEHP in urinary pathway analysis. Bisphenol A (BPA) and other pollutants demonstrated anti-androgenic effects (adverse effects on endocrine function), suggesting that there should be a better method of measuring endocrine disruption that could distinguish between phthalates and pollutants. DEHP was found to be the primary contributor of phthalate exposure through food consumption. The other methods of exposure were shown to have lesser contributions as pathways for endocrine disruptors. It was also shown that other exposure pathways besides dietary were more pertinent for the exposure of phthalates in young adults. 6.2% of adults showed a significant level of phthalate exposure, which is considered to be high, and 25% of children showed a significant level of phthalate exposure. This difference demonstrates that children are more susceptible to phthalate exposure than adults. These results of high-level exposure were shown for DEHP, DiBP, and DnBP.
Dewalque et al. [16] evaluated phthalate concentrations of five different phthalates in Belgium—DEP, DnBP, DiBP, BBzP, and DEHP in urinary pathway analysis. Bisphenol A (BPA) and other pollutants demonstrated anti-androgenic effects (adverse effects on endocrine function), suggesting that there should be a better method of measuring endocrine disruption that could distinguish between phthalates and pollutants. DEHP was found to be the primary contributor of phthalate exposure through food consumption. The other methods of exposure were shown to have lesser contributions as pathways for endocrine disruptors. It was also shown that other exposure pathways besides dietary were more pertinent for the exposure of phthalates in young adults. 6.2% of adults showed a significant level of phthalate exposure, which is considered to be high, and 25% of children showed a significant level of phthalate exposure. This difference demonstrates that children are more susceptible to phthalate exposure than adults. These results of high-level exposure were shown for DEHP, DiBP, and DnBP.Over the past decade, environmental groups, consumers, and government legislation have pushed companies to search for phthalate alternatives. Regardless of the alternatives, in order to successfully phase out phthalate plasticized PVC, substitutes must be easy to process with conventional equipment, stand up to the required mechanical properties, and be manufactured at reasonable prices. When selecting an alternative, manufacturers must often make trade-offs by sacrificing some desired material properties for others that are deemed priorities. For example, it is possible to use less environmentally detrimental polymers such as EVA as alternatives to PVC, but they do not provide mechanical properties as desirable as PVC’s [42].
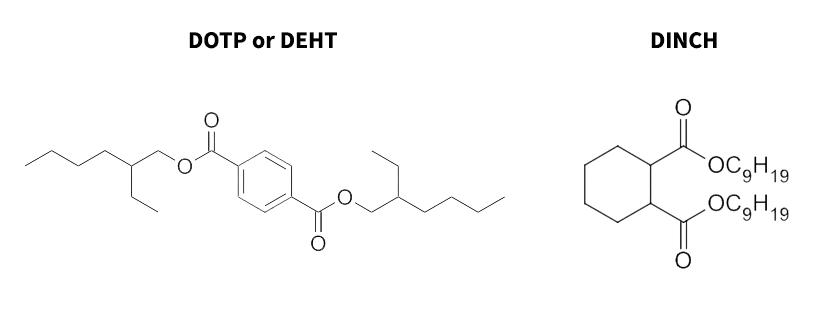
The need for alternatives for phthalates has led manufacturers to create alternatives that share many properties of the phthalate plasticizers without creating endocrine disruption. According to one of the studies conducted by Gray et al. [23], di(2-ethylhexyl) terephthalate (DEHT, see the following left figure) is considered to be a phthalate alternative that has no disruption in sexual development in male mice relative to DEHP. This can be due to the structure of DEHT. DEHT is a terephthalate, which has ester groups on opposite sides of the phenyl ring as opposed to the groups being adjacent to each other. This hinders the ability of DEHT to bind hormone receptors and/or metabolize to a monoester [23]. According to Daniel Schmidt of the Department of Plastics Engineering at the University of Massachusetts, Lowell, DINCH (see the following right figure) is another phthalate alternative that can be used as an alternative to DINP and that is commonly used in wires due to its high molecular weight, which makes it less likely to migrate out of plastic. His recommendation for manufacturing plasticizer alternatives was to avoid aromatic rings, which can lead to endocrine disruption.
The trend to find phthalate alternatives has led environmental groups to create guides that assist electronics companies in eliminating hazardous substances from their products. The most commonly sought-after alternatives are thermoplastic elastomers and bio-based plasticizers. In 2010, a new family of phthalate-free plasticizers was used for wire insulation and jacketing. These plasticizers have shown superior performance and are also made from almost completely renewable feedstocks [43]. However, they remain to be widely accepted.
Thermoplastics, unlike phthalates, can be used in injection molding for small geometries (less than 2 mm) and complex shapes. This enables manufacturers to design smaller and lighter-weight hardware. For example, polycarbonate/acrylonitrile butadiene styrene (PC/ABS) blends have been used in electronic enclosures due to their high modulus, ductility, heat resistance, and impact strength, and are relatively inexpensive. Compositions of thermoplastic elastomers (TPEs) include thermoplastic olefins (TPE-O), copolyamides (COPA), copolyesters (COPE), styrenic compounds (TPE-S), thermoplastic polyurethanes (TPE-U), and vulcanizates (TPE-V) [44]. TPE-V is more flexible and more resistant to extreme temperatures, unlike PVC, which shrinks (Sarlink TPE-V).
 TPEs can be extruded, injection-molded, and thermoformed. These are the popular processing techniques that make TPEs easy to use for manufacturing. Medical products made in theblow/fill/seal process can use TPEs for high-temperature manufacturing, whereas for other plastics such as polyethylene there is a temperature limit of around 121 °C. High-performance TPEs can be over-molded (over-molding eliminates failure of parts by creating chemical bonds between the plastic substrate and the TPE) onto rigid parts. This helps increase the longevity of parts and cost efficiency in manufacturing. Some TPEs can stretch up to 10 times their original length without permanent deformation, and while most TPEs can withstand 400 to 1,000 psi in tension, the wide range of TPE formulations allows some to reach tensile strengths of 5,000 psi [45][46].
TPEs can be extruded, injection-molded, and thermoformed. These are the popular processing techniques that make TPEs easy to use for manufacturing. Medical products made in theblow/fill/seal process can use TPEs for high-temperature manufacturing, whereas for other plastics such as polyethylene there is a temperature limit of around 121 °C. High-performance TPEs can be over-molded (over-molding eliminates failure of parts by creating chemical bonds between the plastic substrate and the TPE) onto rigid parts. This helps increase the longevity of parts and cost efficiency in manufacturing. Some TPEs can stretch up to 10 times their original length without permanent deformation, and while most TPEs can withstand 400 to 1,000 psi in tension, the wide range of TPE formulations allows some to reach tensile strengths of 5,000 psi [45][46].
According to a report from the University of Massachusetts [7], examples of chemical phthalate alternatives include citrates, sebacates, adipates, and phosphates. Like phthalates, chemical alternatives are typically not chemically bound to the plastic they are added to. Therefore, the alternatives can also migrate out and adversely affect the environment and human health. The major health concerns include respiratory illnesses such as asthma as well as skin and eye irritation. However, environmental effects of chemical alternatives can also include adverse effects on aquatic life such as fish, algae, and crustaceans. In addition, several chemical alternatives do not easily biodegrade and accumulate in the environment.
One class of alternative plasticizers to phthalates is bio-based plasticizers. Bio-based alternatives can be made from plant materials such as corn, soy, rice, wheat, and linseed, which makes them much less threatening to animal life. Seven out of the eleven given examples in a report [7] on bio-based alternatives are listed as biodegradable and compostable. Health concerns for these alternatives include skin and eye irritation, respiratory illnesses, and adverse effects on the nervous system. While bio-based alternatives are made from environmentally friendly materials, they are also made from GMOs (genetically modified organisms). Since it is unclear what effects GMOs may have on the environment, it is conceivable that bio-based plasticizers may have unintended effects on the natural environment.
In a study conducted by Benaniba and Massardier-Nageotte [5], epoxidized sunflower oil (ESO) was used as a bio-based co-plasticizer in PVC. The study discussed the effects of ESO when it is used with DEHP on the plasticized material. With 25 wt% of plasticizer, the hardness of the material consisting of DEHP and ESO is lower than the material that uses only DEHP (testing was conducted using the Shore A and D hardness test). In addition, the tensile strength of the plasticized material consisting of both ESO and DEHP was lower than the DEHP plasticized material (tensile strength testing was conducted through a traction test at room temperature at a speed of 20 mm min-1 using a 0.5 KN load cell). Migration was another characteristic that was measured through weight reduction at high temperatures. It was found that compositions consisting of high levels of DEHP and low levels of ESO had higher weight losses. Plasticizer migration was found to be proportional to the concentration, the temperature, the composition, and the nature of the model leachate. Moderate improvements in the measured properties of the plasticized PVC were shown at low levels until ESO was 25% incorporated. The ESO co-plasticizer was shown to be more environmentally friendly than phthalate plasticizers, and with the ideal composition, it can produce improved mechanical properties.
Different countries and regions have their own ways of regulating the use of dangerous chemicals in industry. While phthalate use is not controlled in many developing countries, certain entities such as the European Union and the U.S. government have developed legislation, although still limited, to address health concerns over phthalates.
|
Country |
Substance |
Limit (by weight) |
Conditions of restriction |
|
European Union: |
DEHP DBP BBP DIBP |
DEHP+DBP+BBP<=0.1% |
The plasticized material in toys and childcare articles. (DIBP can be used only with Authorization.) |
|
European Union: |
DINP DIDP DNOP |
DINP+DIDP+DNOP<=0.1% |
The plasticized material in toys and childcare articles which can be placed in the mouth by children. |
|
European Union: |
DEHP DBP BBP DIBP |
DEHP<=0.1% |
All electrical and electronic equipment: 22 July 2019. Category 8 (medical devices) and Category 9 (monitoring and control equipment): 22 July 2021. |
|
USA: |
DEHP DBP BBP |
DEHP<=0.1% |
Children's toys and childcare articles for children under 3. |
|
DINP
DIDP DNOP |
DINP<=0.1% |
All three phthalates are interim banned, so the restriction is applied to children's toy that only can be placed in a child's mouth and childcare articles. |
|
|
Japan: |
DEHP DBP BBP |
DEHP<=0.1% |
Synthetic resin, mainly composed of PVC, for children’s toys. |
|
DINP DIDP DNOP |
DINP<=0.1% |
Synthetic resin, mainly composed of PVC, for children’s toys are intended to contact with mouth (excluding pacifiers and teething rings) and the toys intended for children under three. Pacifiers and teething rings without using PVC as raw material. |
|
|
Canada: |
DEHP DBP BBP |
DEHP<=0.1% |
The vinyl in toys and childcare articles. |
|
DINP DIDP DNOP |
DINP<=0.1% |
The vinyl in any part of toys and childcare products that can be placed in mouth for children under 4. |
|
|
Brazil |
DEHP DBP BBP |
DEHP<=0.1% |
All children’s toys and childcare articles for children under 3. |
|
DINP DIDP DNOP |
DINP<=0.1% |
All children’s toys and child care articles that can be placed in children’s mouth. |
|
|
Argentina |
DEHP DBP BBP DINP DIDP DNOP |
DEHP+DBP+BBP<=0.1% |
All children’s toys and childcare articles for children under 3. |
|
DINP+DIDP+DNOP<=0.1% |
All children’s toys and child care articles that can be placed in children’s mouth under 3. |
||
|
Australia/ New Zealand: |
DEHP |
DEHP<=0.1% |
All children’s toys and child care articles that can be placed in children’s mouth under 3. |
Notes:
DEHP: Bis (2-ethylhexyl) phthalate; DBP: Dibutyl phthalate; BBP: Benzyl butyl phthalate; DINP: Di-‘isononyl’ phthalate; DIDP: Di-‘isodecyl’ phthalate; DNOP: Di-n-octyl phthalate;
DIBP: Diisobutyl phthalate
Childcare article: Any product intended to facilitate sleep, relaxation, hygiene, the feeding of children or sucking on the part of children.
Many articleas have been addressing the concerns with phthalates on the health and environment because some phthalates have been considered to affect hormone receptor proteins and enzymes, which are involved in the synthesis or activation of hormones. Some of the articles addressed on the concerns with health and environments about the leakage of phthalates Others were focused on the migration from phthalates-contained to non-phthalates and updating restrictions on the phthalates. The articles are categorized by various topics, and the number in the square bracket ahead the authors for each article represents the reference number cited in this website.
[4] P. Ventrice, D. Ventrice, E. Russo, and G. De Sarro, "Phthalates: European regulation, chemistry, pharmacokinetic and related toxicity. Environmental Toxicology and Pharmacology," Vol. 36, No. 1, pp. 88-96, 2013.
[10] S. Gärtner, M. Balski, M. Koch, and I. Nehls, “Analysis and migration of phthalates in infant food packed in recycled paperboard,” Journal of Agricultural and Food Chemistry, Vol. 57, No. 22, pp. 10675-10681, 2009.
[39] C. Hogue, “U.S. to restrict five phthalates in children’s products,” Chemical & Engineering News Global Enterprise, Vol. 95, No. 43, p. 13, 2017.
[48] B. E. Erickson, “European Union further restricts four phthalates,” Chemical & Engineering News Global Enterprise, Vol. 95, No. 26, p. 15, 2017.
[2] D. Liang, T. Zhang, H. Fang, and J. He, "Phthalates biodegradation in the environment," Applied Microbiology and Biotechnology, Vol. 80, No. 2, pp. 183-198, 2008.
[6] H. Frederiksen, N. Skakkebaek, and A. Andersson, "Metabolism of phthalates in humans," Molecular Nutrition & Food Research, Vol. 51, No. 7, pp. 899-911, 2007.
[11] K. Boisen, M. Kaleva, K. Main, H. Virtanen, A. Haavisto, I. Schmidt, M. Chellakooty, I. Damgaard, C. Mau, M. Reunanen, N. Skakkebaek, and J. Toppari, "Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries," The Lancet, Vol. 363, No. 9417, pp. 1264-1269, 2004.
[12] E. Huyghe, P. Plante, and P. Thonneau, "Testicular Cancer Variations in Time and Space in Europe," European Urology, Vol. 51, No. 3, pp. 621-628, 2007.
[13] R. Preikša, B. Žilaitienė, V. Matulevičius, N. Skakkebæk, J. Petersen, N. Jørgensen, and J. Toppari, "Higher than expected prevalence of congenital cryptorchidism in Lithuania: a study of 1204 boys at birth and 1 year follow-up," Human Reproduction, Vol. 20, No. 7, pp. 1928-1932, 2005.
[14] K. Boisen, M. Chellakooty, I. Schmidt, C. Kai, I. Damgaard, A. Suomi, J. Toppari, N. Skakkebaek, and K. Main, "Hypospadias in a Cohort of 1072 Danish Newborn Boys: Prevalence and Relationship to Placental Weight, Anthropometrical Measurements at Birth, and Reproductive Hormone Levels at Three Months of Age," The Journal of Clinical Endocrinology & Metabolism, Vol. 90, No. 7, pp. 4041-4046, 2005.
[15] N. Skakkebaek, N. Jorgensen, K. Main, E. Meyts, H. Leffers, A. Andersson, A. Juul, E. Carlsen, G. Mortensen, T. Jensen, and J. Toppari, "Is human fecundity declining?" International Journal of Andrology, Vol. 29, No. 1, pp. 2-11, 2006.
[16] L. Dewalque, C. Charlier, and C. Pirard, "Estimated daily intake and cumulative risk assessment of phthalate diesters in a Belgian general population," Toxicology Letters, Vol. 231, No. 2, pp. 161-168, 2014.
[17] A. Martino-Andrade and I. Chahoud, "Reproductive toxicity of phthalate esters," Molecular Nutrition & Food Research, Vol. 54, No. 1, pp. 148-157, 2010.
[18] A. Schecter, M. Lorber, Y. Guo, Q. Wu, S. H. Yun, K. Kannan, L. S. Birnbaum, "Phthalate Concentrations and Dietary Exposure from Food Purchased in New York State," Environmental Health Perspectives, Vol. 121, No. 4, pp. 473-479, 2013.
[19] W. Wang, X. Xu, and C. Fan, "Health hazard assessment of occupationally di-(2-ethylhexyl)-phthalate-exposed workers in China," Chemosphere, Vol. 120, pp. 37-44, 2015.
[20] R. A. Rudel, D. E. Camann, J. D. Spengler, L. R. Korn, and J. G. Brody, “Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust,” Environmental Science & Technology, Vol. 37, No. 20, pp. 4543-4553, 2003.
[21] S. K. Ritter, “Phthalates’ structural truths,” Chemical & Engineering News Archive, Vol. 93, No. 25, pp. 19-20, 2015.
[23] L. Gray, J. Ostby, J. Furr, M. Price, D. N. Veeramachaneni, and L. Parks, "Perinatal exposure to the phthalates DEHP, BBP, and DINP, but Not DEP, DMP, or DOTP, alters sexual differentiation of the male rat," Toxicological Sciences, Vol. 58, No. 2, pp. 350-365, 2000.
[24] S. Sivaramanan, "E-Waste Management, Disposal and Its Impacts on the Environment," Universal Journal of Environmental Research & Technology, Vol. 3, No. 5, pp. 1-7, 2013.
[25] K. Brigden, J. Webster, I. Labunska, and D. Santillo, "Toxic Chemicals in Computers Reloaded," Greenpeace, 2007.
[26] L. Patton, "CPSC Staff toxicity review of 17 phthalates," United States Consumer Product Safety Commission, 2016.
[27] U.S. Food and Drug Administration, "Ingredients – Phthalates," Center for Food Safety and Applied Nutrition, 2016.
[28] S. Nordbrand, "Out of Control: E-waste trade flows from the EU to developing countries," SwedWatch, 2009.
[29] W. Liu, C. Shen, Z. Zhang, and C. Zhang, "Distribution of Phthalate Esters in Soil of E-Waste Recycling Sites from Taizhou City in China. Bulletin of Environmental Contamination and Toxicology," Vol. 82, No. 6, pp. 665-667, 2009.
[30] A. Saoji, "E-waste management: an emerging environmental and health issue in India," National journal of medical research, Vol. 2, No. 1, pp. 107-110, 2012.
[32] American Chemical Society, “Phthalates in Sediments,” Environmental Science & Technology, Vol. 29, No. 12, p. 535A, 1995.
[33] Z. Xie, R. Ebinghaus, C. Temme, R. Lohmann, A. Caba, and W. Ruck, “Occurrence and air−sea exchange of phthalates in the arctic,” Environmental Science & Technology, Vol. 41, No. 13, pp. 4555-4560, 2007.
[34] J. Ejlertsson, M. Alnervik, S. Jonsson, and B. H. Svensson, “Influence of water solubility, side-chain degradability, and side-chain structure on the degradation of phthalic acid esters under methanogenic conditions,” Environmental Science & Technology, Vol. 31, No. 10, pp. 2761-2764, 1997.
[36] B. Hileman, “Phthalates in toys,” Chemical & Engineering News Archive, Vol. 82, No. 40, p. 11, 2004.
[40] B. Erickson, "Regulators and Retailers Raise Pressure on Phthalates," Chemical & Engineering News, Vol. 93, No. 25, p. 11-15, 2015.
[47] G. Sadeghi, E. Ghaderian, and A. O'Connor, "Determination of Dioctyl phthalate (DEHP) concentration in polyvinyl chloride (PVC) plastic parts of toothbrushes," The Downtown Review, Vol. 1, No. 2, n/a, 2015.
[5] M. Benaniba and V. Massardier-Nageotte, "Evaluation effects of biobased plasticizer on the thermal, mechanical, dynamical mechanical properties, and permanence of plasticized PVC," Journal of Applied Polymer Science, Vol. 118, No. 6, pp. 3499-3508, 2010.
[7] Lowell Center for Sustainable Production, "Phthalates and Their Alternatives: Health and Environmental Concerns," University of Massachusetts, 2011.
[22] N. Kambia, A. Farce, K. Belarbi, B. Gressier, M. Luyckx, P. Chavatte, and T. Dine, "Docking study: PPARs interaction with the selected alternative plasticizers to di(2-ethylhexyl) phthalate," Journal of Enzyme Inhibition and Medicinal Chemistry, Vol. 31, No. 3, pp. 448-455, 2016.
[42] A. Lindström and M. Hakkarainen, "Environmentally friendly plasticizers for poly(vinyl chloride)—Improved mechanical properties and compatibility by using branched poly(butylene adipate) as a polymeric plasticizer," Journal of Applied Polymer Science, Vol. 100, No. 3, pp. 2180-2188, 2006.
[43] J. Kuczynski and D. Boday, "Bio-based materials for high-end electronics applications," International Journal of Sustainable Development & World Ecology, Vol. 19, No. 6, pp. 557-563, 2012.
[44] Teknor Apex, "Thermoplastic Elastomers: The Softest Materials Solving Your Hardest Problems," Teknor Apex, 2016.
[45] J. Kutka, "Thermoplastic Elastomer (TPE) Market Growth Continues," Machine Design, 2009.
[46] Y. Chen, A. Kushner, G. Williams, and Z. Guan, "Multiphase design of autonomic self-healing thermoplastic elastomers," Nature Chemistry, Vol. 4, No. 6, pp. 467-472, 2012.
[1] Greenpeace, "The dangerous chemicals in electronic products," Greenpeace, 2016.
[3] ECPI, "Orthophthalates," 2014.
[8] Apple, "Environmental Responsibility Report: 2016 Progress Report," Covering Fiscal Year 2015. Apple, 2016.
[9] M. Cobbing and T. Dowdall, "Green Gadgets: Designing the Future-The path to greener electronics," Greenpeace International, 2014.
[31] J. Puckett, E. Hopson, and M. Huang, "Disconnect: Goodwill and Dell, Exporting the Public’s E-Waste to Developing Countries," Basel Action Network, 2016.
[35] European Commission, "RoHS Amendment adding Phthalates to Restricted Substances is Published," European Commission, 2016.
[37] L. Patton, "Toxicity Review of two less common phthalates and one phthalate alternative for the CHAP," United States Consumer Product Safety Commission, 2011.
[38] L. Patton, "CPSC Staff toxicity review of 17 phthalates," United States Consumer Product Safety Commission, 2011.
[41] U.S. Food and Drug Administration, "Guidance for industry limiting the use of certain phthalates as excipients in CDER-regulated products," The Administration, 2012.
For questions or concerns regarding phthalates or CALCE's research into phthalate safety and alternatives, contact Professor Michael Pecht:
301-405-5323 | pecht@calce.umd.edu